Microbiology of environmental samples
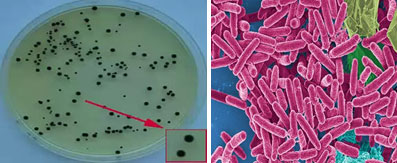
Environmental media (air, surfaces) present permanent but variable microbiological contamination in time and space. Faced with this ubiquitous contamination, the protection of products, vulnerable individuals and analytical conditions can only be considered in a controlled microbial environment. It is therefore necessary to assess the risks of contamination and to manage these risks in an appropriate and consistent manner. It is a question, in fact, of permanently controlling and regularly monitoring the microbiological quality of all the environmental media. This monitoring is based on various methods, sampling and analysis of micro-organisms in the environment

The LCAE microbiology laboratories: Guarantor of Quality

- Experience of more than 30 years
- Availability of this activity at the technical centers of TUNIS, SFAX and SOUSSE of the LCAE
- Accreditation of the microbiology laboratory of food products and water of TUNIS since 2004 (about 17 years), by the French Accreditation Committee COFRAC then the National Accreditation Council TUNAC .
- Participation in inter-comparison networks: RAEMA since 2003; BIPEA since 2007; AGLAE: since 2012; PHE since 2017.
- Regular monitoring of the validity of the results by applying an internal control program throughout the performance of the analyzes
Type of Analysis:
Type of Products:
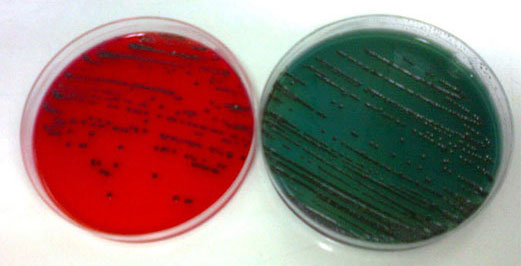
Environmental samples
- Air quality
- Surface quality
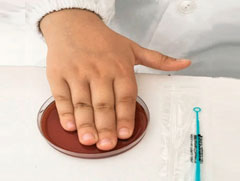
- Quality of handprints
Our skills :
- TUNAC Accredited Laboratory since 2004
- Accreditation number: 1-0002
- Scope of Accreditation: Technical appendix ortunac.tn
- Inter-comparison trials: RAEMA - PHE- AGLAE